In February, 150 federal agents descended on The Scooter Store’s national headquarters in New Braunfels searching for evidence of Medicare fraud. The case shines a light on the hazards of direct-to-consumer advertising by medical equipment and prescription drug companies.
 TV ads have driven the nearly $1 billion U.S. market for power wheelchairs and scooters, showing smiling seniors on a motorized scooter enjoying the views of the Grand Canyon, fishing on a pier and with their grandchildren at a baseball game. Doctors and lawmakers say the ads create the false impression that scooters are a convenient means of transportation rather than a medical necessity.
TV ads have driven the nearly $1 billion U.S. market for power wheelchairs and scooters, showing smiling seniors on a motorized scooter enjoying the views of the Grand Canyon, fishing on a pier and with their grandchildren at a baseball game. Doctors and lawmakers say the ads create the false impression that scooters are a convenient means of transportation rather than a medical necessity.
Medicare will pay for 80 percent of the (approved) amount for a motorized scooter if the senior has a doctor’s order stating the patient cannot use a cane or walker or can’t operate a manual wheelchair. Members of Congress say the ads lead to hundreds of millions of dollars in unnecessary spending by Medicare and that 80 percent of the scooters and power wheelchairs Medicare buys go to people who don’t meet the requirements.
In 2005, the U.S. Justice Department sued The Scooter Store, alleging that its advertising enticed seniors to obtain power scooters paid for my Medicare, and it then sold patients more expensive scooters that they didn’t want or need. The company settled that case in 2007 for $4 million and agreed to periodic reviews.
In 2011, government auditors estimated that the company received between $47 million and $88 million in improper payments for scooters. The company took no action to repay the money until February 2012, when the Health and Human Services inspector general threatened to bar the company from Medicare, which comprises 75 percent of its income. Since the raid, the company has laid off most of its 1,800 workers and is operating on a skeleton crew.
Direct-to-consumer advertising is legal in only two nations — the United States and New Zealand. Until the mid-1980s, companies gave information about medical equipment and prescription drugs only to doctors and pharmacists. When these professionals thought it appropriate, they gave that information to their patients.
In the mid-1980s, drug and equipment companies began advertising directly to the consumers. Because Food and Drug Administration guidelines required detailed information on usage and risks, advertising was effectively limited to print. In 1997, the FDA relaxed its regulations so that only major statements of the risks and benefits were required, thus crumbling the barrier to radio and TV broadcast advertising, the costs of which have soared about 20 percent per year and now top several billion dollars.
Research on direct-to-consumer advertising has been limited, but it indicates that the effects are both positive and negative.
I confess that for many years I felt the practice should be outlawed. I felt it set up unrealistic expectations on the part of the patient and that it made the job harder for the health care professional to deal with patients coming in saying they want this drug or that. Advertising amounts to sales pitches that misinforms patients, overemphasizes drug or equipment benefits and promotes new drugs before safety profiles were fully known. It “medicalizes” natural conditions leading to drug over-utilization, leads to inappropriate prescribing, strains relationships with health care providers, wastes appointment time, is not rigorously regulated and increases costs. (Think The Scooter Store mess here.)
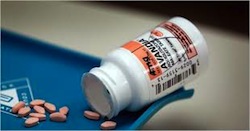 And let’s not forget the scandal surrounding the diabetes drug, Avandia. In 2006, it was the biggest-selling diabetes drug in the world with sales topping $3.2 billion. Yet a study estimated that from 1999 to 2009, more than 47,000 people taking Avandia needlessly suffered a heart attack, stroke or heart failure, or died. In 2010, Senate investigators found that GlaxoSmithKline spent years hiding from regulatory authorities clear indications that Avandia increased heart risks, and the company took a $2.3 billion liability charge. Europe suspended its sales, while U.S. patients would be allowed access to the drug only if their doctors attested that they had tried every other diabetes medicine and that their patients were made aware of the risks.
And let’s not forget the scandal surrounding the diabetes drug, Avandia. In 2006, it was the biggest-selling diabetes drug in the world with sales topping $3.2 billion. Yet a study estimated that from 1999 to 2009, more than 47,000 people taking Avandia needlessly suffered a heart attack, stroke or heart failure, or died. In 2010, Senate investigators found that GlaxoSmithKline spent years hiding from regulatory authorities clear indications that Avandia increased heart risks, and the company took a $2.3 billion liability charge. Europe suspended its sales, while U.S. patients would be allowed access to the drug only if their doctors attested that they had tried every other diabetes medicine and that their patients were made aware of the risks.
Nonetheless, over time I’ve come to the conclusion that banning a manufacturer from marketing its products won’t pass constitutional scrutiny, as we’ve got this First Amendment freedom-of-speech thing. On the bright side, the advertising can inform and empower patients, encourage patients to contact a clinician, promote dialogue with medical providers, strengthen a patient’s relationship with a clinician, encourage patient compliance, reduce underdiagnosis and undertreatment of conditions, may remove stigma associated with certain diseases and possibly encourage product competition and lower prices.
Still, every time I see one of these ads I find myself muttering “Eeww.” The practice is unseemly in an advanced society.
 Austin, Texas
Austin, Texas